EVALUATION OF THE EFFICACY AND SAFETY OF COMPLEX THERAPY OF ACUTE RESPIRATORY DISEASES OF ADENOVIRAL ETIOLOGY IN YOUNG PEOPLE
2 Military Medical Academy Named After S.M. Kirov (Russian Federation, 194044, St. Petersburg, Academician Lebedev St., 6).
ABSTRACT
An important current epidemiological phenomenon is the simultaneous circulation of different types and strains of viruses, including influenza viruses, as well as the presence of mixed infections — influenza and other acute respiratory viral infections in one patient. Adenovirus infections occupy a leading position in the general structure of acute respiratory viral infections, especially in periods when influenza rarely occurs. Bearing in mind the particular clinical course of adenovirus infection, the principal challenge of antiviral therapy, in addition to relieving the manifestations at the height of the disease, is to suppress adenovirus persistence and reduce the frequency of development of late pneumonias and a protracted and recurrent clinical course. A retrospective analysis of case histories where interferon inducers and interferons were used to treat adenoviral diseases in young people established that Kagocel, an inducer of the «late» interferons, significantly decreased the incidence of pneumonia and a protracted and recurrent course compared with the baseline (pathogenetic) therapy.
Keywords: Adenoviral disease, antiviral therapy, interferon, tilorone, meglumine acridоnacetate, Kagocel.
INTRODUCTION
Adenovirus infection accounts for a significant share in the etiological structure of ARVI in young people in organized groups. 1, 2, 3 The course of acute respiratory diseases of adenoviral etiology is characterized by the frequent development of purulent tonsillitis, a protracted and recurrent course, and adenovirus-associated pneumonia which can be fatal. 4, 5 Therefore, the search for effective therapy and prevention of adenovirus infections is an important objective of military medicine.
Direct and indirect-acting antiviral agents are currently used to treat acute respiratory diseases, including adenoviral diseases. 6, 7, 8 We have previously shown that direct-acting antiviral drugs (ribavirin and umifenovir) significantly reduce the duration of the pronounced clinical manifestations of adenoviral disease. 9 However, these drugs have not demonstrated a significant effect on the incidence of pneumonia or a protracted and recurrent course. This is probably due to the predominant effect of etiotropic agents on the adenovirus replication stage which is accompanied by pronounced clinical manifestations. A protracted and recurrent course of adenovirus infection, as well as late pneumonias, are caused by the development of the integrative phase and a prolonged persistence of adenoviruses in the lymphoid tissue. It should be expected that the use of indirect-acting (mainly immunotropic) antiviral drugs (interferon and interferon inducers) will reduce the incidence of complications of ARVI, including those of adenoviral etiology. 10
Study aim: to assess the clinical efficacy of antiviral drugs with an indirect (immunomodulating) action in the treatment of adenoviral diseases in young people.
MATERIALS AND METHODS
The medical histories of 292 patients with adenoviral diseases of moderate severity who underwent inpatient treatment at the Clinic of Infectious Diseases of the S.M. Kirov Military Medical Academy (St. Petersburg) were analyzed. The diagnosis of «adenovirus infection» was verified by virological (virus isolation and/or PCR) and serological (IgM and/or IgG CFT and EIA) laboratory methods. 11, 12, 13 The analysis included the medical histories of only those patients who were admitted in the first 48 hours after the onset of the pronounced clinical manifestations, with an uncomplicated course by the time of admission and prescription of the drug (an interferon inducer or interferon). The analysis also included the medical histories of patients with an adenovirus infection verified by laboratory methods who received only pathogenetic therapy in the same period (control group). As a result, five comparison groups were created: group 1 (control, 96 patients) received only the background therapy (bed rest, diet, multivitamins and pathogenetic agents); group 2 (47 patients), in addition to the background therapy, received human leukocyte interferon, 250,000 IU, inhalation aerosol with medium dispersion once a day for 3 days; group 3 (58 patients), in addition to background therapy, received meglumine acridоnacetate, 150 mg tablets orally, 4 tablets on days 1 and 2, then 2 tablets on days 4, 6, 8. Course 1.5-3 g (10-20 tablets); group 4 (44 patients), in addition to background therapy, received an inducer of the «early» interferons tilorone, 125 mg tablets orally, 1 tablet per day on days 1 and 2 of treatment, then 1 tablet every 48 hours on days 4 and 6 of administration; group 5 (47 patients), in addition to background therapy, received an inducer of «late» interferons (Kagocel) 12 mg tablets orally, 2 tablets 3 times a day on the first two days of treatment, 1 tablet 3 times a day on days 3 and 4 of treatment (4-day course).
The criteria for the clinical, as well as therapeutic and preventive efficacy of the adenoviral therapy were as follows: the average duration of the intoxication syndrome and fever; the mean day on which the maximum fever was recorded; the average duration of the respiratory syndromes and cough, non-respiratory syndromes (conjunctivitis and tonsillitis), as well as a decrease in the frequency of development of a protracted, recurrent course of the disease and penumonia complications.
RESULTS
The compared groups were comparable in the time of initiation of therapy, sex, age, as well as the severity of the intoxication syndrome and febrile response on the first day of antiviral therapy. The number of observed cases of adenoviral disease in each comparison group was representative (more than 30). Thus, a mathematical statistical analysis was conducted and parametric and nonparametric statistical methods were applied in order to identify differences.
The mean duration of manifestations of the intoxication syndrome and fever, mean total duration of febrile response, mean day of the maximum body temperature during therapy, mean duration of respiratory syndromes, cough and some non-respiratory syndromes (conjunctivitis and tonsillitis) are presented in Table 1.
| Criterion | Antiviral drug (n — number of observations) | ||||
|---|---|---|---|---|---|
| Background (n=96) | Interferon (n=47) | Meglumine acridonacetate (n=58) | Tilorone (n=44) | Kagocel (n=47) | |
| Duration of intoxication, days | 5.6±1.21 | 4.3±2.51 | 4.5±2.11 | 5.1±1.66 | 4.2±1.79 |
| Maximum body temperature, day | 2.8±1.84 | 2.2±1.13 | 2.2±1.49 | 2.1±1.37 | 1.8±1.03 |
| Duration of fever, days | 3.4±1.98 | 1.6±0.88* | 3.0±1.22 | 2.6±1.59 | 2.4±1.42 |
| Total duration of fever, days | 5.8±2.22 | 3.4±1.77 | 4.9±2.66 | 4.8±2.91 | 3.9±1.75 |
| Rhinitis, days | 7.5±2.48 | 9.3±2.24 | 7.5±2.95 | 7.3±2.28 | 4.1±2.27* |
| Pharyngitis, days | 5.8±1.91 | 4.5±2.17 | 5.1±1.17 | 4.7±2.53 | 3.4±2.80 |
| Laryngitis, days | 5.6±1.21 | 5.1±2.25 | 5.0±2.54 | 5.4±2.70 | 3.5±1.57 |
| Tracheitis, days | 5.1±1.21 | 3.4±1.43 | 3.4±2.29 | 4.0±2.28 | 3.0±0.61 |
| Bronchitis, days | 9.0±3.48 | 8.7±2.82 | 8.7±4.87 | 7.5±3.62 | 4.3±3.86 |
| Cough, days | 8.2±3.42 | 8.6±2.64 | 8.0±6.16 | 8.2±3.53 | 3.5±1.32* |
| Conjunctivitis, days | 8.2±3.03 | 9.0±2.96 | 7.0±2.94 | 8.6±2.91 | 4.7±2.81 |
| Tonsillitis, days | 6.0±2.64 | 5.6±2.41 | 5.5±2.44 | 9.6±2.13 | 3.5±1.82 |
| Note: * - significantly (p < 0.05) less than the background therapy | |||||
The «early» interferon inducers (meglumine acridonacetate and tilorone) did not significantly affect the duration of general infectious syndromes (intoxication, fever and total length of the febrile response), and respiratory syndromes (rhinitis, pharyngitis, laryngitis, tracheitis, bronchitis), cough and non-respiratory syndromes (acute conjunctivitis and acute tonsillitis). The dynamics of individual symptoms that demonstrated a significant difference (p <0.05) from the background therapy is presented in Fig. 1.
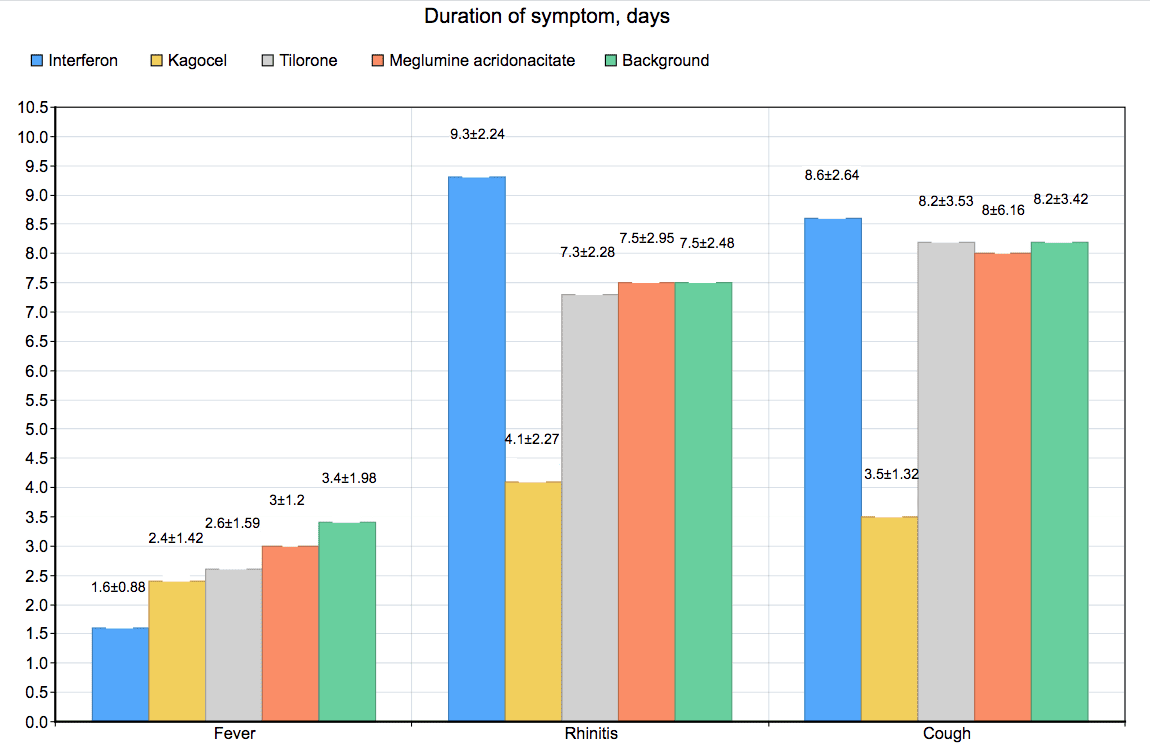
Note: * - significantly (p <0.05) less than the background therapy
The use of interferon demonstrated a significant difference from the background therapy only in the duration of fever (1.6 ± 0.88 days vs. 3.4 ± 1.98 days, p <0.05), while there was no difference between the time of development of the maximum temperature and the total length of febrile response between the groups.
The use of Kagocel, an inducer of the «late» interferons, significantly decreased the duration of rhinitis (4.1 ± 2.27 days vs. 7.5 ± 2.48 days, p <0.05), and cough (3.5 ± 1, 32 days vs. 8.2 ± 3.42 days, p <0.05) compared with the baseline therapy.
Acute respiratory viral infections, including adenoviral diseases, with uncomplicated purulent tonsillitis, sinusitis and pneumonia, are known to be characterized by spontaneous recovery.
In connection with this, and the previously identified features of the course of clinical adenovirus infection, the most significant indicators of the clinical efficacy of antiviral drugs in the treatment of patients with ARVI of adenoviral etiology are a reduction in the incidence of complications and a protracted and recurrent course of the disease.
To assess the therapeutic and preventive efficacy of interferons and IFN inducers compared to the background therapy according to the proposed criteria, the frequency of complications (pneumonia, purulent sinusitis, tubo-otitis, recurrence of HSV infection), and a protracted and recurrent course were calculated and statistically compared using Pearson’s χ2 test.
The incidence (%) of pneumonia, sinusitis, tubo-otitis, and recurrence of HSV infection in patients with adenoviral diseases, as well as of a protracted and recurrent course of ADVI in the treatment of patients with adenovirus infections taking interferon and interferon inducers is shown in Fig. 2.
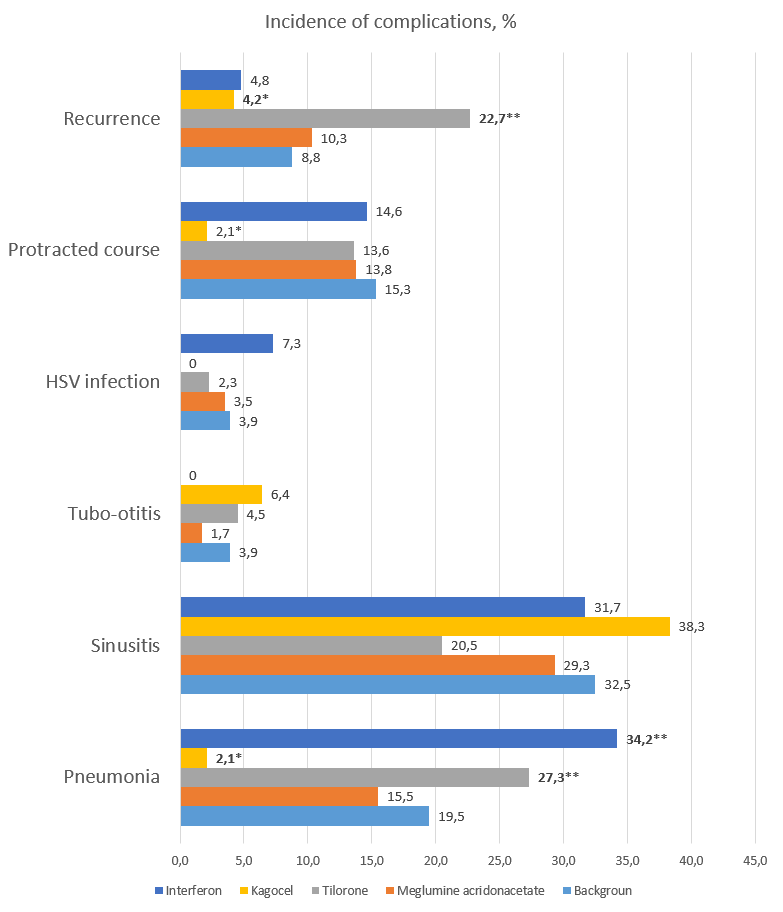
Note: * - significantly (p < 0.05) less in comparison with the background therapy; ** - significantly (p <0.05) more compared to the background therapy.
A comparative statistical analysis of the frequency of development of a complicated, protracted and recurrent course demonstrated that oral administration of meglumine acridonacetate, tilorone, and interferon inhalations did not significantly decrease the incidence of complications or a protracted and recurrent course. Moreover, in comparison with the background therapy, the use of tilorones significantly raised the incidence of pneumonia and a recurrent course, while interferons only increased pneumonia. In the first case, this may be due to the fact that tilorones stimulate substantial production of early alphainterferon, in the second case — to the fact that interferon was administered by inhalation and with deep forced breaths of patients. The latter probably led to a downward spread of adenoviruses to the lower respiratory tract and lungs.
The use of the «late» interferon inducer Кagocel in the treatment of patients with adenoviral diseases significantly reduced the incidence of pneumonia by 9.28 times (2.1% vs. 19.5%, p <0.05) as compared with the standard ARVI therapy, as well as a protracted course by 7.28 times (2.1% vs. 15.3%, p <0.05) and recurrence by 2.1 times (4.2% vs. 8.8%, p <0.05). Also, as noted above, there was a significant decrease in the duration of rhinitis (by 3.4 days, p <0.05) and cough (by 4.7 days, p <0.05) compared with the baseline therapy. An analysis of the conducted therapy identified no adverse reactions. It is worth noting that the duration of therapy with the «late» interferon inducer Kagocel was 4 days, in contrast to the use of tilorone — 6 days, and meglumine acridonacetate — 8 days.
DISCUSSION
Taking into account the data of previously conducted studies, direct-acting antiviral agents (ribavirin and umifenovir) remain the drugs of choice for the relief of the clinical syndromes during the phase of pronounced clinical manifestations of medium and severe adenovirus diseases as part of complex therapy. 9 Based on the results of the conducted study, the drugs of choice to prevent the development of pneumonia, and a protracted and recurrent course of adenoviral disease are polyphenol derivatives, e.g. Kagocel.
It should be expected that combined antiviral therapy with a simultaneous use of direct (ribavirin or umifenovir) and indirect (Kagocel) acting drugs will have a synergistic therapeutic and preventive effect. 10 For instance, combined therapy of influenza A (H1N1) pdm09 virus has shown that concurrent use of direct-acting antiviral agents (oseltamivir and umifenovir) and Kagocel, an interferon inducer that stimulates the patient’s innate immunity, is more effective than monotherapy with a direct-acting antiviral drug, which was expressed in a more rapid relief of the disease symptoms and a reduction in the incidence of complications. 14 Meanwhile, the inclusion of an indirect-acting antiviral agent (including a late interferon inducer) in the complex therapy of ARVI and influenza is not limited to viral etiology. This is important in the current epidemic situation with a simultaneous circulation of different types and strains of viruses, including influenza viruses, as well as mixed infections with influenza and other respiratory viruses in one patient. Numerous studies of Kagocel have confirmed its efficacy, regardless of the etiology of the identified respiratory pathogen. 15 Such a positive effect of Kagocel on these parameters may be due to the stimulation of production of interferon (probably mainly gamma) by polyphenol derivatives, which led to an accelerated elimination of virus-infected epithelial and lymphoid cells in most patients.
CONCLUSIONS
As the conducted studies have demonstrated, the inducers of «early» interferons (meglumine acridonacetate and tilorone) did not significantly affect the duration of the general infectious, respiratory and non-respiratory syndromes in the treatment of adenovirus infection. Administration of interferon led to a significant difference with the background therapy only in the duration of fever. The use of the «late» interferon inducer Kagocel in the treatment of patients with adenoviral diseases significantly reduced the duration of rhinitis (by 3.4 days, p <0.05) and cough (by 4.7 days, p <0.05) compared with the baseline therapy, and, unlike the «early» inducers, significantly decreased the incidence of pneumonia by 9.28 times (2.1% vs 19.5%, p <0.05), a protracted course by 7.28 times (2.1% against 15.3%, p <0.05) and a recurrent course by 2.1 times (4.2% vs. 8.8%, p <0.05). The drug combined well with the conducted symptomatic therapy and demonstrated a high safety profile. The obtained results unambiguously confirm the clinical efficacy of Kagocel in the treatment of acute respiratory viral infections and are in full agreement with the published studies of other authors. 14, 15, 16
REFERENCES
- Lvov N.I., Zhdanov K.V., Lobzin Yu.V. et al. Clinical and epidemiological significance of adenovirus infection in military personnel. Voen. Med. Zh, 2013; 8: 19-25.
- Lvov N.I., Pisareva M.M., Maltsev O.V. et al. The features of the etiological structure of ARVI in certain age and occupational groups of the population of St. Petersburg in the epidemic season 2013-2014. Journal of Infectology, 2014; 6(3): 62-70.
- Zhdanov K.V., Lvov N.I., Maltsev O.V. et al. Main Aetiological Features of Acute Respiratory Viral Diseases in Young People of Draft Age and Conscripts During the 2013-2014 Epidemic Season. International Review of the Armed Forces Medical Services, 2016; 82(2): 58–63.
- Ivanov V.V., Kharitonov M.A., Grozovsky Yu.R. Severe virus-associated pneumonia in military personnel. Bulletin of the Russian Military Medical Academy, 2015; 1: 146-152.
- Lvov N.I., Sominina A.A., Zhdanov K.V., Lobzin Yu.V. Features of the clinical course of acute respiratory diseases caused by adenoviruses of epidemically significant serotypes. Journal of Infectology, 2014; 6(2): 5-11.
- Bulgakova V.A., Poromov A.A., Grekova A.I. et al. Pharmaco-epidemiological study of the course of influenza and other ARVI in high-risk groups. Therapeutic archive, 2017; 89(1): 62-71.
- Zhdanov K.V., Zakharenko S.M., Likhopoenko V.P., Lvov N.I. Diagnosis and treatment of acute respiratory diseases: Methodology manual, St. Petersburg; VMedA, 2012; 21.
- Lyutov V.V., Blinda I.V., Gribova L.N. et al. Military doctor’s action algorithms on admission of patients with influenza, ARI, pneumonia, generalized meningococcal infection: Methodology guidelines, St. Petersburg; VMedA, 2016; 32
- Lvov, N.I., Zhdanov K.V., Lobzin Yu.V., Maleev V.V. Experience in the use of antiviral drugs in acute respiratory diseases of adenoviral etiology. Infectious diseases, 2013; 11(4): 65-71.
- Lobzin Yu.V., Lvov N.I. Interferon inducers in the therapy of acute respiratory diseases: Problems and Perspectives. Voen. Med. Zh., 2001; 11: 41-50.
- Ageeva M.R., Yatsyshina S.B., Lvov N.I. Advantage of PCR in the diagnosis of respiratory adenovirus infection. Laboratory Service, 2016; 5(3): 35-36.
- Amosova I.V., Timoshicheva T.A., Sverlova M.V. et al. Use of micro cell culture enzyme immunoassay and modified immunofluorescence in the diagnosis of adenovirus infection. Clinical laboratory diagnostics, 2017: 62(4): 230-235.
- Yanina M.A., Komissarov A.B., Lvov N.I., Osidak L.V. Determination of adenovirus genotypes in samples obtained from patients with ARVI. Molecular diagnostics, 2014; 1: 336-337
- Popov A.F., Shchelkanov M.Yu., Dmitrenko K.A., Simakova A.I. Сombined therapy of influenza with antiviral drugs with a different mechanism of action in comparison with monotherapy. J. Pharm. Sci. & Res, 2018; 10(2): 357-360.
- Fazylov V.Kh., Sitnikov I.G., Malyshev N.A., Silina E.V., Shevchenko S.B., Yeganyan G.A., Korsantiya B.M., Groppa L.G. The effect of antiviral therapy on the incidence of bacterial complications and the prescription of systemic antibacterial drugs in patients with influenza and ARVI (the results of a cohort international observational study). Antibiotics and chemotherapy, 2016; 61(11-12): 39-47.
- Sologub T. V., Tsvetkov V. V. Kagocel in the treatment of influenza and acute respiratory viral infections: an analysis and systematization of data on the results of preclinical and clinical studies. Therapeutic archive, 2017; 8: 113-119
Article Received on 20 July 2018,
Revised on 10 August 2018,
Accepted on 30 August 2018,
DOI: 10.20959/wjpr201816-13307
*Corresponding Author Malyshev Nikolay Alexandrovich
A.I. Yevdokimov Moscow State University of Medicine and Dentistry (Russian Federation, 127473, Moscow, Delegatskaya St., 20, p. 1).
